Password Reset
Forgot your password? Enter the email address you used to create your account to initiate a password reset.
Forgot your password? Enter the email address you used to create your account to initiate a password reset.
Surgery to treat specific forms of drug-resistant epilepsy (DRE) has proven highly efficacious in well-selected patients with focal epilepsies. Advances in diagnostic approaches and imaging modalities, coupled with new surgical techniques and technologies, have expanded and evolved markedly during the last 20 years, bolstered by robust longitudinal data from clinical trials.
Compassionate, comprehensive multidisciplinary patient care–from diagnosis to optimal treatments to long-term follow-up care–led by highly trained epileptologists working in tandem with surgical partners and supported by other medical experts leads to quality improvements in care and better outcomes for epilepsy patients. Such is the operational methodology at the UPMC Comprehensive Epilepsy Center.
However, nationally, only a small percentage of patients with focal DRE who are or could be excellent candidates for surgical treatment to markedly reduce or even completely control their seizures have surgery or access to surgical options in their geographic region. Current data in the literature show that for every one candidate that has epilepsy surgery, there are possibly 25 to 50 candidates who do not. The reasons are many.
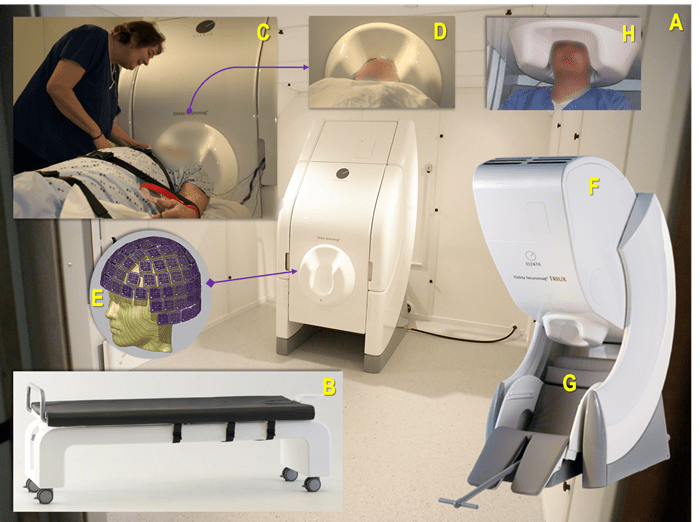
Figure 1 (A-H). A: The UPMC MEG Center's existing MEG device (Vectorview, Elekta Neuromag Oy, Helsinki, Finland) within a magnetically shielded room set up for a recording in a supine position using a proprietary bed (B); C: The first UPMC MEG technologist and coordinator, Anna Haridis (2006), preparing a patient with epilepsy for a MEG-EEG recording while ensuring patient safety and comfort, and that the head is positioned optimally within the helmet (D) containing the MEG sensors configured as depicted in image E; F: A more recent version of a MEG device (Triux) positioned for recording a patient comfortably while in a seated position (G), with their head in an optimal head position (H). Thanks are extended to Mr. N.M. for volunteering for photo H.
Similarly, the adoption and use of magnetoencephalography (MEG) to assist the neurologist and neurosurgeon and complement their use of other techniques and technologies in the localization and presurgical mapping of seizure foci to resect, and eloquent areas of the brain to avoid, has lagged since the technology was first invented in the 1970s and first cleared by the U.S. Food and Drug Administration (FDA) for use in epilepsy in the 1986, followed by its status in 2002 as a fully accepted clinical service by the U.S. Centers for Medicare and Medicaid (CMS).
UPMC Comprehensive Epilepsy Center Director Anto Bagić, MD, PhD, FAES, FACNS, who also serves as Chief of the Epilepsy Division in the Department of Neurology and is the Director of the UPMC MEG Epilepsy Program, guest-edited a special thematic issue of the Journal of Clinical Neurophysiology (JCN) that is focused on MEG – its history, development, use, and evolution as a clinical tool; gaps and deficiencies in its wider adoption by clinicians and health care systems; and, perhaps most importantly, educating fellow neurologists and neurosurgeons who specialize in epilepsy about the clinical utility and benefits of MEG, advocating for its broader dissemination into the field of epilepsy (see below for more information on the MEG issue of JCN and Dr. Bagić’s contributions).
Dr. Bagić is a long-time and early proponent for the use of MEG in epilepsy. UPMC and the University of Pittsburgh were relatively early adopters of the technology in 2005 upon his arrival, and were the first institutions in the surrounding multistate area to begin using MEG in clinical practice. Dr. Bagić is a passionate and vocal advocate for MEG's clinical use and the study of its benefits in treating patients with drug-resistant epilepsy, and more broadly in his advocacy for expanded access to surgical treatments and follow-up care for all epilepsy patients. His prior work with MEG on the national and international stages helped generate the world’s first MEG Clinical Practice Guidelines in 2011, published by the American Clinical MEG Society (ACMEGS). (Dr. Bagić is a founder and inaugural board member of ACMEGS.)1-5
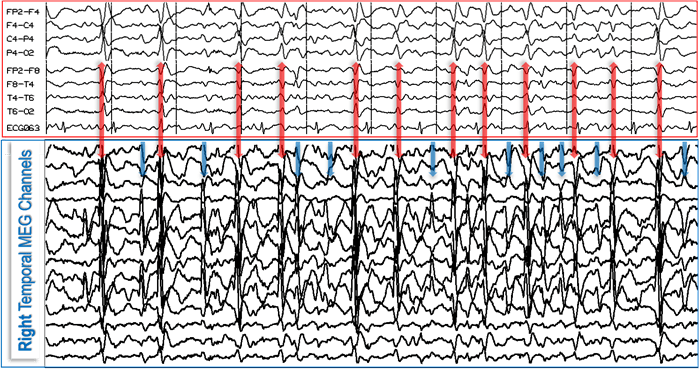
Figure 2. MEG and EEG are complementary neurophysiological methods that reveal two physical manifestations, magnetic (MEG) and electric (EEG), of the same physiologic or pathologic processes in the brain and provide the highest yield when combined as illustrated. In this case, the EEG (red box, top) reveals 12 epileptiform discharges (red double-headed vertical arrows) that also are seen on MEG (blue box, bottom); however, the MEG picks up at least 10 additional epileptiform discharges (blue vertical arrows) which are absent from, or minimally reflected on, the EEG.
“MEG provides unique (non-redundant) information noninvasively but remains vastly underutilized in epilepsy and beyond. I do not believe there is any substantive debate to be argued on that fact. The data from surveys we conducted that form the basis for two studies in the current edition of the JCN support this rationale and the existing body of evidence to that effect. The larger issue that we all must grapple with is that there are far too many individuals in this country (and the world) that could benefit from surgery for their DRE but, for one reason or another, cannot access a potentially curable treatment. When we fail to use the most powerful tools available to us to treat our patients–MEG or otherwise–we are engaged in behavior that is far from benign neglect. To be blunt, tens of thousands of people suffer in innumerable ways because surgery is out of reach for them. In our noble aspiration to optimize epilepsy care, we–the epilepsy community–must capitalize on a powerful synergy between overcoming underutilization of epilepsy surgery and accountable clinical utilization of MEG”, says Dr. Bagić.
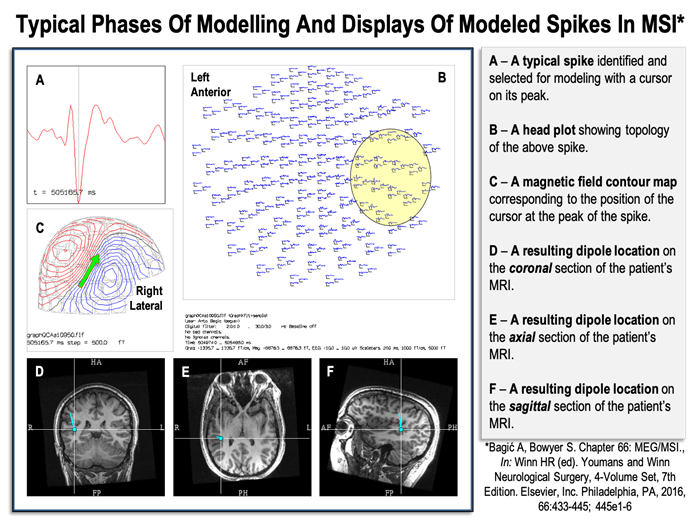
Figure 3. Typical phases of modeling and displays of modeled spikes in Magnetic Source Imaging (MSI).
Much like the ubiquitously available electroencephalography (EEG) records the electrical potentials reflecting neuronal activity in the brain, MEG records the minute magnetic fields generated by the same electrical currents of neurons. Every electrical current – large or small – generates a magnetic field that can be measured under the right circumstances. For comparison, the magnetic fields generated by cellular activity in the human brain are roughly 10-14 tesla. The magnetic field generated within the earth’s core is approximately 10-5 tesla–about 50 to 100 million times as strong as the magnetic fields emanating from the human brain A garden-variety MRI scanner generates a magnetic field of 1.5 tesla, which is billions of times more powerful.5 The sensors used in MEG devices are called superconducting quantum interference devices (SQUIDS) were first invented in late 1960s. Because of the exquisite sensitivity of SQUIDS, they are considered by some physicists to be the most sensitive and powerful recording devices yet invented.
In order for MEG devices to function and detect the infinitesimally small magnetic fields generated in the human brain, they must be housed in special magnetically shielded rooms that are able to isolate the subjects sufficiently from all ambient magnetic fields – be they from the earth’s core, walking humans with magnetic or magnetized objects, opening and closing metal doors, wheeling hospital carts, etc. For MEG and the SQUIDS that make up the device to operate and achieve their superconducting state, they must be continuously cooled to close to absolute zero (4.2°K) with liquid helium.
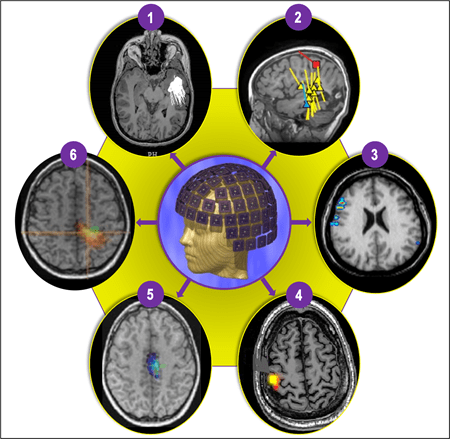
Figure 4 (1-6). The most commonly used and extensively clinically validated form of MEG is magnetic source imaging (MSI) (1 and 2), which is sometimes synonymous with MEG, particularly in epileptology. MSI is a method in which models of the magnetic sources are represented with a dot (or small triangle or square) and tail, where a dot represents a “dipole” (source) location, and a tail represents the source direction. MSI can be used to represent various interictal (yellow), ictal (blue), and functional landmarks (e.g., sensory cortex = red square), creating a neuronavigational map (2) that can be delivered to the planning and neuronavigational software used during the planning and operating phases, respectively. Depending on the type of clinical or research application, MEG data can be processed using a multitude of other techniques that can produce somewhat differently appearing but increasingly similar data displays. Four other more commonly used methods include Current Density Distribution Techniques (3); Minimum Norm Estimate (MNE) (4); Multiple Signal Classification (MUSIC) (5); and Synthetic Aperture Magnetometry (SAM) and excess kurtosis [SAM(g2)] (6).
MEG and EEG are complementary tools deployed simultaneously in the field of epilepsy because of their varying sensitivities and abilities to capture cerebral sources of seizure activity in different orientations and depths- they provide the highest yield of data when used simultaneously in combination within the full diagnostic armamentarium of imaging modalities and other technologies to capture and interpret neurophysiologic data – data that can assist the neurologist, epileptologist, and neurosurgeon, in more precisely understanding the nature and location of a person’s seizure activity in the brain.
At present, MEG is used for only two reimbursable indications in clinical practice: to obtain clinical data for the presurgical planning (and clinical hypothesis confirmation) in cases of DRE, and to aid in the precise mapping of eloquent cortices of the brain to avoid during surgical resection or ablation of tissues in cases of epilepsy or other operable lesions. In fact, MEG has applications in any type of brain surgery where the identification of eloquent cortices is of paramount concern. As a research tool, MEG is used to study a range of conditions in the medical field (e.g., Alzheimer’s disease, brain injuries, brain plasticity, cognitive processes) apart from other scientific uses. MEG also is increasingly replacing the more invasive WADA test used for language and memory lateralization because of its more robust capabilities of localization beyond just the gross brain hemisphere detection capable with WADA.
“MEG is a noninvasive test – a passive listening device; it exposes its subjects to no radiation and is pain-free. It enables us to examine the human brain as if it were stripped of its surrounding tissues – completely exposed,” says Dr. Bagić. Preparation and pre-screening for MEG exams is more involved because all potential interferences or introduction of magnetic sources into the special magnetically-shielded room MEG scanners are housed in must be identified and eliminated to preserve the integrity of the acquired scan data.
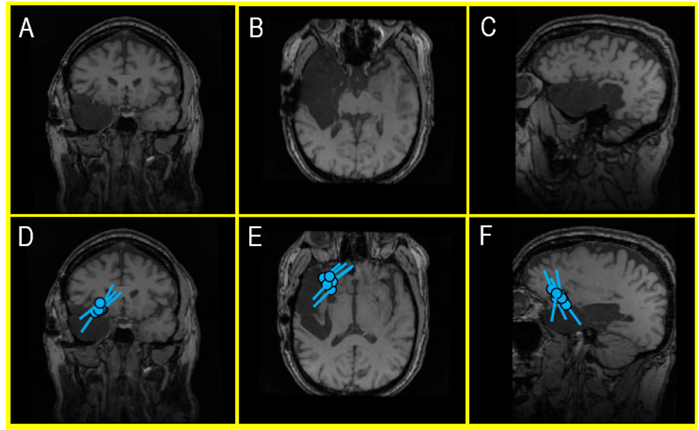
Figure 5 (A-F). An MRI of a recent external MEG referral patient (A, B, C) showing a large cavity following a right temporal lobectomy that ultimately failed to control the patient’s seizures with antiseizure medications (ASM). A second surgery was considered, but an EEG – in the context of a skull defect that distorts electrical signals – provided a misleading indication that the patient’s seizures continued to be produced in the remaining part of the right temporal lobe. However, a MEG – which is unaffected by surgically changed anatomy of the brain and skull – identified a very localized seizure focus within the inferior frontal gyrus (D, E, F). This area became the new center of attention and landmark for planning an invasive EEG monitoring prior to a new, targeted surgical intervention. During the clinical care of epilepsy patients, this kind of nonredundant information is invaluable for developing a treatment plan and the surgical outcomes.
In a significant portion of cases, the literature shows the ability of MEG to provide additional, non-redundant information about a patient’s epilepsy that can, at times, alter a person’s surgical candidacy. A MEG test can help find potential surgical candidates who may have to that point been ruled out for surgery. MEG can assist the clinician to find otherwise hidden candidates.
For decades, MEG has existed as a clinical tool available for neurologists and epileptologists, but broad adoption has not occurred. According to Dr. Bagić, even among practicing neurologists and even epileptologists, there is a profound lack of education and understanding of MEG's use and benefits, even in centers with their own MEG instruments (there are more than 100 MEG devices in clinical use worldwide).
"The fact that neurologists and neurosurgeons have little knowledge of the clinical utility of MEG to better assist and confirm our clinical hypotheses for cases of DRE, and to ultimately use the information we derive from it to plan more targeted and, we hope, more successful surgeries is the impetus for the journal edition. We must begin with education," says Dr. Bagić.
In addition to his work on guest-editing the special MEG edition of JCN, Dr. Bagić single-authored or first-authored five of the 14 articles in it related to his and other’s ongoing research and clinical experiences with MEG, with a focus on clinical practice in epilepsy and preoperative brain mapping.6-10
The first of Dr. Bagić and colleagues' new papers, "Utilization of MEG Among the US Epilepsy Centers: A Survey-Based Appraisal," provides data from a survey of National Association of Epilepsy Centers about their clinical use of MEG. Two hundred twenty-five centers were invited to participate in the anonymous, web-based survey. Seventy centers in total participated, 61 of which are designated as level 4 epilepsy centers.
The survey's main goals were to investigate how MEG use patterns may be affected by the characteristics of a center and its clinical patient volumes and ascertain the center's clinical value placed on MEG in its practice.
“We did not find anything relevant in the data relative to a specific characteristic in a center that would lead to higher MEG use. However, we did find that centers with higher surgical volumes were more likely to have a MEG instrument at their institution. They also claimed that they would use MEG more if they had more complex patients. Interestingly, the volume of patients admitted to the Epilepsy Monitoring Unit (EMU) did not correlate with higher levels of MEG use,” says Dr. Bagić.
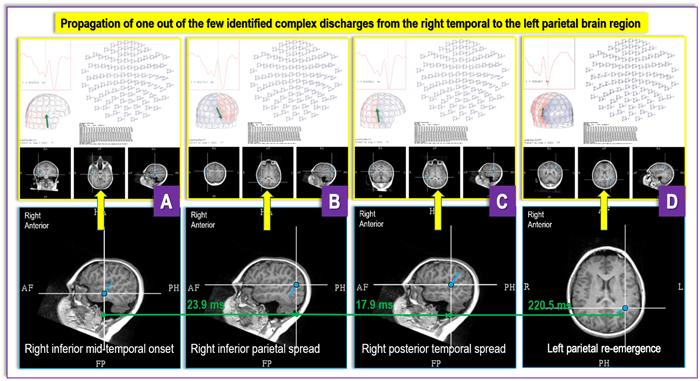
Figure 6 (A-D). Figure 6 depicts findings from a recent DRE MEG referral from UPMC Children’s Hospital of Pittsburgh for a case with “generalized” and “multifocal” EEG findings, where a MEG revealed an irritative zone (an area of the brain producing interictal discharges) in the base of the right temporal lobe (A) that propagates over the ipsilateral hemisphere (B and C) and then reemerges contralaterally (D). These findings provide critical new insight into the patient’s epilepsy and open a possibility that the seizures' focus is contained within the inferior aspects of the right temporal lobe. The “generalized” and “multifocal” discharges seen on an EEG may represent differently – chronologically and topologically – propagated focal discharges. If ultimately confirmed through invasive testing, these MEG findings would radically change the prospect that this patient is a viable surgical candidate with the opportunity for a positive outcome after surgery.
Future research will be needed to better understand what factors may drive a center to adopt MEG or, conversely, what factors may prohibit it?
"We also must overcome a larger issue in referral patterns of patients to comprehensive epilepsy centers for evaluation and potential surgical intervention. As is usually the case, there are many factors at work, but again, we must find ways to remove the barriers patients face to receiving optimal care. Of course, not every patient is a surgical candidate, but for those who are or potentially may be upon further evaluation, we are underserving their needs," says Dr. Bagić.
In the other articles contributed or co-authored by Dr. Bagić, there is a wealth of information for the clinician new to MEG or those interested in a more in-depth understanding of its origins, indications, and implications for clinical practice. In the paper titled," The Wisdom and Vision From the ACMEGS Inaugural Decade,” Dr. Bagić and colleagues provide a comprehensive history and chronology tracking the development of MEG as technology from its roots in the physics community at the beginning of the 20th century to the seminal work of David Cohen in the 1960s, to the current state of the technology and its modern-day advances, use, and advocacy through ACMEGS.
For the clinician with little to no exposure to MEG, Dr. Bagić and his co-authors have penned an article summarizing the more common evidence-based indications for the use of MEG in epilepsy surgery for intractable cases. The 10 common indications reviewed in the article are accompanied by sample patient cases and brief histories to illustrate how MEG use could benefit the case.
“I think it bears repeating that the vast majority of practicing neurologists, epileptologists, and neurosurgeons specializing in epilepsy surgery have little practical clinical MEG experience. Including real-world cases to accompany the more common indications for MEG that we summarize in the article provides concrete examples for readers to delve into and bolster their understanding of its potential benefits,” says Dr. Bagić.
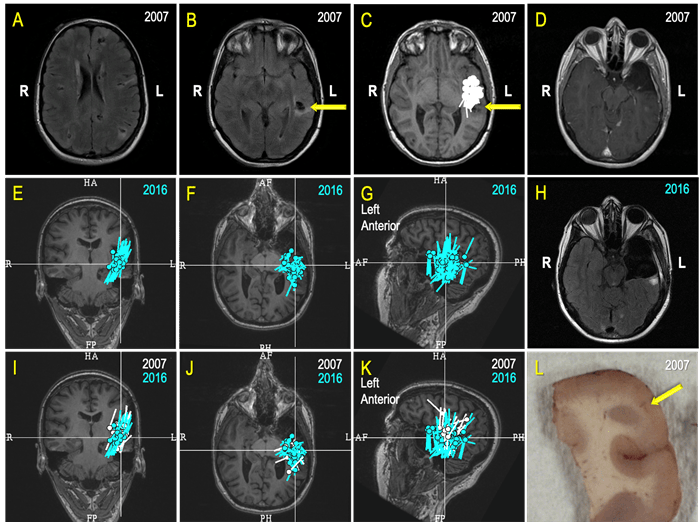
Figure 7. A Case Review (A-L): A 26-year-old college graduate began experiencing seizures a few times a week 10 years prior (at age 16) as she was about to acquire a driver’s license. The patient failed several antiseizure medications (ASMs) and aged out of pediatric care at UPMC Children's. An operation to cure her epilepsy was her constant desire, but the prospect of surgery was a very challenging and risky proposition because she has a multifocal disease – Tuberous sclerosis (A, B). After a comprehensive evaluation, a MEG greatly simplified planning for an invasive monitoring by revealing a seizure focus close to a left temporal, partially calcified tuber (C, yellow arrow on B, C & D; 2007). Ultimately, a possible degree of the left anterior temporal resection, limited by the language functions close to the tuber (D; 2007), led to seizure freedom and "new, great life.” Unfortunately for this patient, seven years later, in 2014, she again began to have seizures. A new set of investigations, including a MEG, revealed “a cluster” indicative of an active seizure focus in the “same area” as before (E, F, G). The patient underwent a modern type of invasive monitoring (stereo-EEG) planned around her MEG findings. She ultimately had a more extensive left temporal surgery (H). In preparation for a multidisciplinary patient management discussion, our team displayed the MEG results from 2007 (white) and 2016 (turquoise) on the same MRI (I, J, K) and established that there was significant overlap. As epileptologists, our team inferred that the patient’s original surgery did not remove the seizure focus but instead only interrupted its manifesting network. While this surgery led to seven years of freedom from seizures, and to quote the patient, a “new, great life," it became apparent that a more complete resection would be necessary to give the patient another opportunity at a seizure-free life. Histopathologic findings within the resected specimen (L) from the second surgery revealed cortical development malformation with Balloon Cells and Rosenthall fibers. The patient experienced occasional seizures during the first year following her second surgery, but she is now enjoying the fourth year of a new seizure-free era. Thus, an accountable and competent incorporation of MEG in the comprehensive multidisciplinary evaluation and treatment of this patient directly contributed to rescuing at least 10 years of this young life from disabling seizures.
A final article co-authored by Dr. Bagić, along with Richard Burgess, MD, PhD, from the Cleveland Clinic, explores usage patterns of MEG in clinical practice among 25 clinical MEG centers. The survey conducted as part of this research shows that during the past decade, the volume of MEG use in the U.S. has reached a plateau and remains a somewhat distant part of the standard of care in the majority of epilepsy centers despite its clinical utility and clinical guidelines for its application.
"Despite its potential MEG just has not broken through into the wider epilepsy community - yet. While that is frustrating for those of us who are proponents of the technology and have seen its abilities to improve outcomes for epilepsy surgery and help expand the true percentage of patients who may ultimately benefit from surgical intervention, our hope from the work in the MEG-specific issue of the JCN is that we will expand opportunities to educate our colleagues and thereby increase the acceptance and use of MEG for the benefit of our epilepsy patients," says Dr. Bagić.
“The amount of effort by my collaborators in this endeavor has been tremendous, and it certainly would not exist without their collective expertise, dedication, and tireless advocacy for broader clinical adoption of MEG in epilepsy and neurosurgery. We have been some of the true believers in the power of MEG from its earliest days. It is my fervent hope that the articles, research, and editorials we all have contributed to this edition inspire our neurology and neurosurgery colleagues around the United States and the world to learn more about MEG in the pursuit of much better care and superior outcomes for our most challenging and complex patients with epilepsies and operable brain lesions.”
Learn more about the UPMC Comprehensive Epilepsy Center, and the UPMC Brain Mapping Center. More about Dr. Bagić’s work and research can be found by visiting his faculty page on the University of Pittsburgh School of Medicine Department of Neurology website.
1. Bagić AI, Knowlton RC, Rose DF, Ebersole JS; For the ACMEGS Clinical Practice Guideline (CPG) Committee. American Clinical Magnetoencephalography Society Clinical Practice Guideline 1: Recording and Analysis Of Spontaneous Cerebral Activity. J Clin Neurophysiol. 2011; 28: 348–354.
2. Burgess RC, Funke ME, Bowyer SM, Lewine JD, Kirsch HE, Bagić AI; For the ACMEGS Clinical Practice Guideline (CPG) Committee. American Clinical Magnetoencephalography Society Clinical Practice Guideline 2: Presurgical Functional Brain Mapping (PFBM) Using MEG Evoked Fields (MEFs). J Clin Neurophysiol. 2011; 28: 355–361.
3. Bagić AI, Knowlton RC, Rose DF, Ebersole JS; For the ACMEGS Clinical Practice Guideline (CPG) Committee. American Clinical Magnetoencephalography Society ClinicalPpractice Guideline 3: MEG-EEG Reporting. J Clin Neurophysiol. 2011; 28: 362–363.
4. Bagić AI, Barkley GL, Rose DF, Ebersole JS; For the ACMEGS Clinical Practice Guideline (CPG) Committee. American Clinical Magnetoencephalography Society Clinical Practice Guideline 4: Qualifications of MEG-EEG Personnel. J Clin Neurophysiol. 2011; 28: 364–365.
5. Singh SP. Magnetoencephalography: Basic Principles. Ann Indian Acad Neurol. 2014; S107-112.
6. Bagić, AI, Burgess, RC. Utilization of MEG Among the US Epilepsy Centers: A Survey-Based Appraisal. J Clin Neurophysiol. 2020; 37:599-605.
7. Bagić AI. SQUIDs Pro Quorum. J Clin Neurophysiol. 2020; 37: 469-470. Editorial
8. Bagić AI, Funke ME, Kirsh HE, Tenney JR, Zillgitt AJ, Burgess RC. The 10 Common Evidence-Supported Indications for MEG in Epilepsy Surgery: An Illustrated Compendium. 2020; 37; 483-497.
9. Bagić AI, Burgess, RC. Clinical Magnetoencephalography Practice in the United States Ten Years Later: A Survey-Based Reappraisal. J Clin Neurophysiol. 2020; 37: 592-598.
10. Bagić AI, Funke ME, Burgess RC. The Wisdom and Vision From the ACMEGS Inaugural Decade. J Clin Neurophysiol. 2020; 37: 471-482.